Jenscare | 2024H1 Newsletter
6-Month Clinical Results of LuX-Valve Plus TRAVEL II Study were Officially Released at New York Valves 2024 and OCC-WCC 2024
The 6-month clinical follow-up of clinical trial TRAVEL II study of LuX-Valve Plus, which was independently developed by Jenscare (9877.HK), were officially published by Prof. Juan F. Granada (Cardiovascular Research Foundation, New York, U.S.) at New York Valves 2024 and by Academician Junbo Ge (葛均波院士, Zhongshan Hospital Affiliated to Fudan University, Shanghai, China) jointed with Prof. Daxin Zhou (周达新教授, Zhongshan Hospital Affiliated to Fudan University, Shanghai, China) and Prof. Fanglin Lu (陆方林教授, Shanghai General Hospital, Shanghai, China) at the 18th Oriental Congress of Cardiology together with the World Congress of Cardiology (OCC-WCC 2024). The astonishing results demonstrated the excellent performance within the unique design and won high appraisals among the attendances.
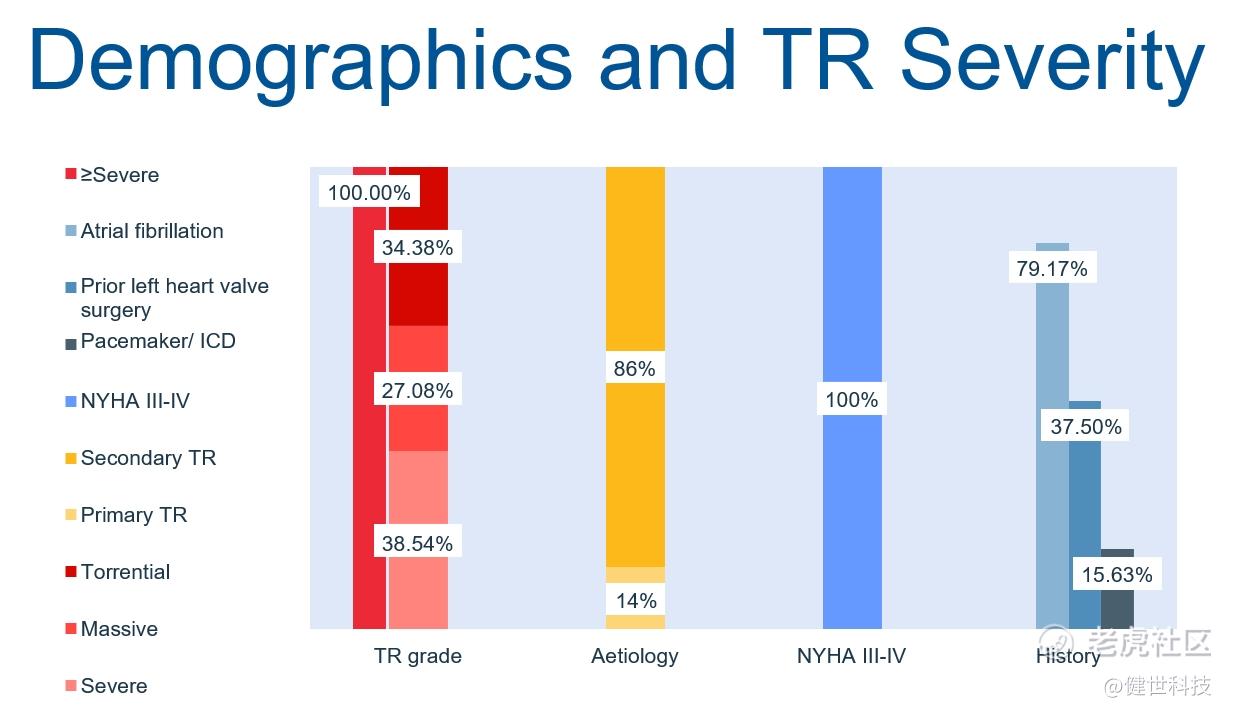
TRAVEL II study is a prospective, multi-center and single-arm clinical trial, which primarily aims to evaluate the acute and long-term safety and efficacy of LuX-Valve Plus in patients with severe tricuspid regurgitation (TR). TRAVEL II clinical trial has enrolled 96 patients from 15 centers in China. The average age of the patients was 71.35 years old with an average STS score of 9.09%. 37.5% of the patients had prior left heart valve surgery, and 15.63% of them had pacemaker/ICD implanted before. Patients were combined with multi other comorbidities, which formed a poor baseline and increased the risks of surgical treatment or difficulties of other interventional therapies.
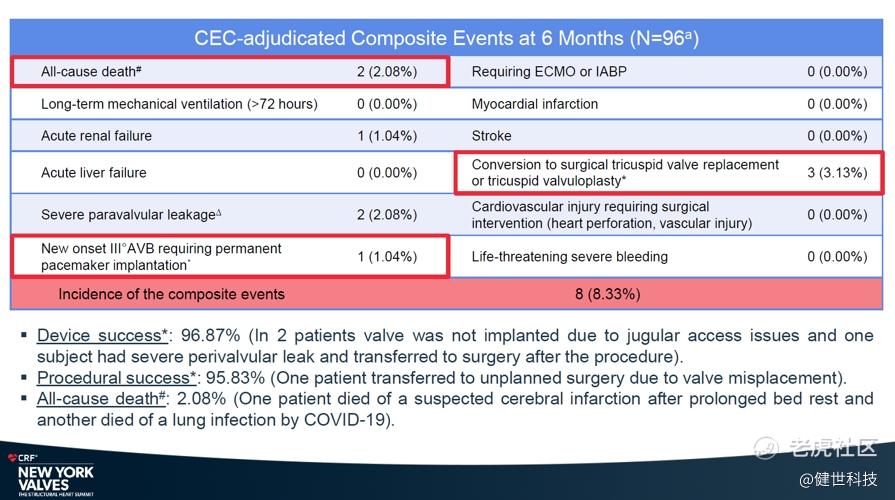
The safety results showed that the incidence of composite events is 8.33%. Incidence of myocardial infarction, stroke, use of ECMO or IABP, acute liver failure, long-term mechanical ventilation (>72 hours), cardiovascular injury requiring surgical intervention (heart perforation, vascular injury), and life-threatening severe bleeding were all 0%. All-cause mortality was only 2.08%. Incidence of acute renal failure, severe paravalvular leakage, and conversion to surgical tricuspid valve replacement or tricuspid valvuloplasty were 1.04%, 2.08%, and 3.13% respectively. Incidence of new onset degree III AVB requiring permanent pacemaker implantation was only 1.04%.
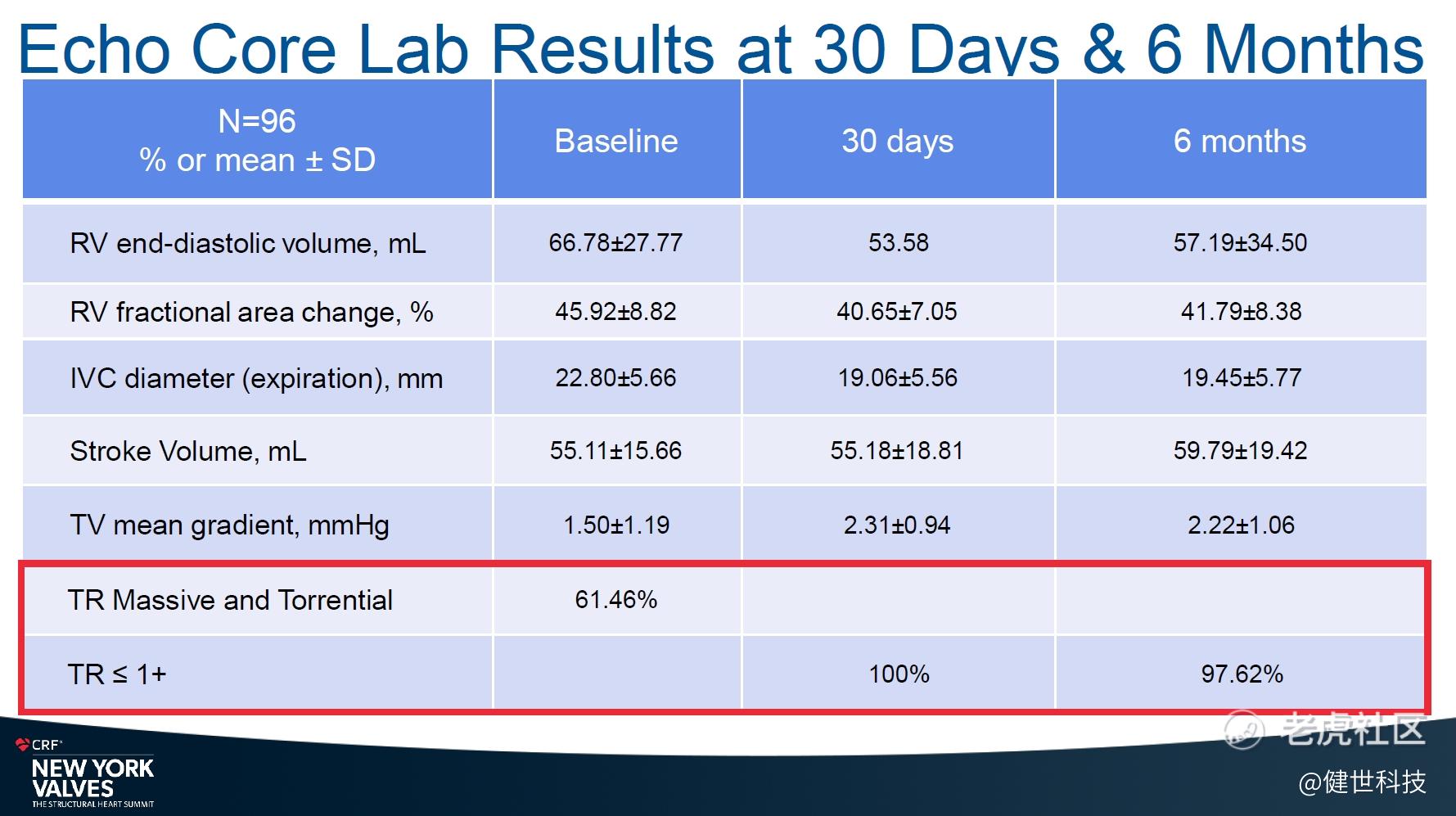
The efficacy results showed that the TR grade, NYHA classification, and QoL (quality of life) improved significantly. 100% and 97.62% of patients showed no moderate or above tricuspid regurgitation (TR) at 30 days and at 6 months, respectively. Meanwhile, the reformation of right heart/ventricle benefits as well according to the Echo Core Lab results.
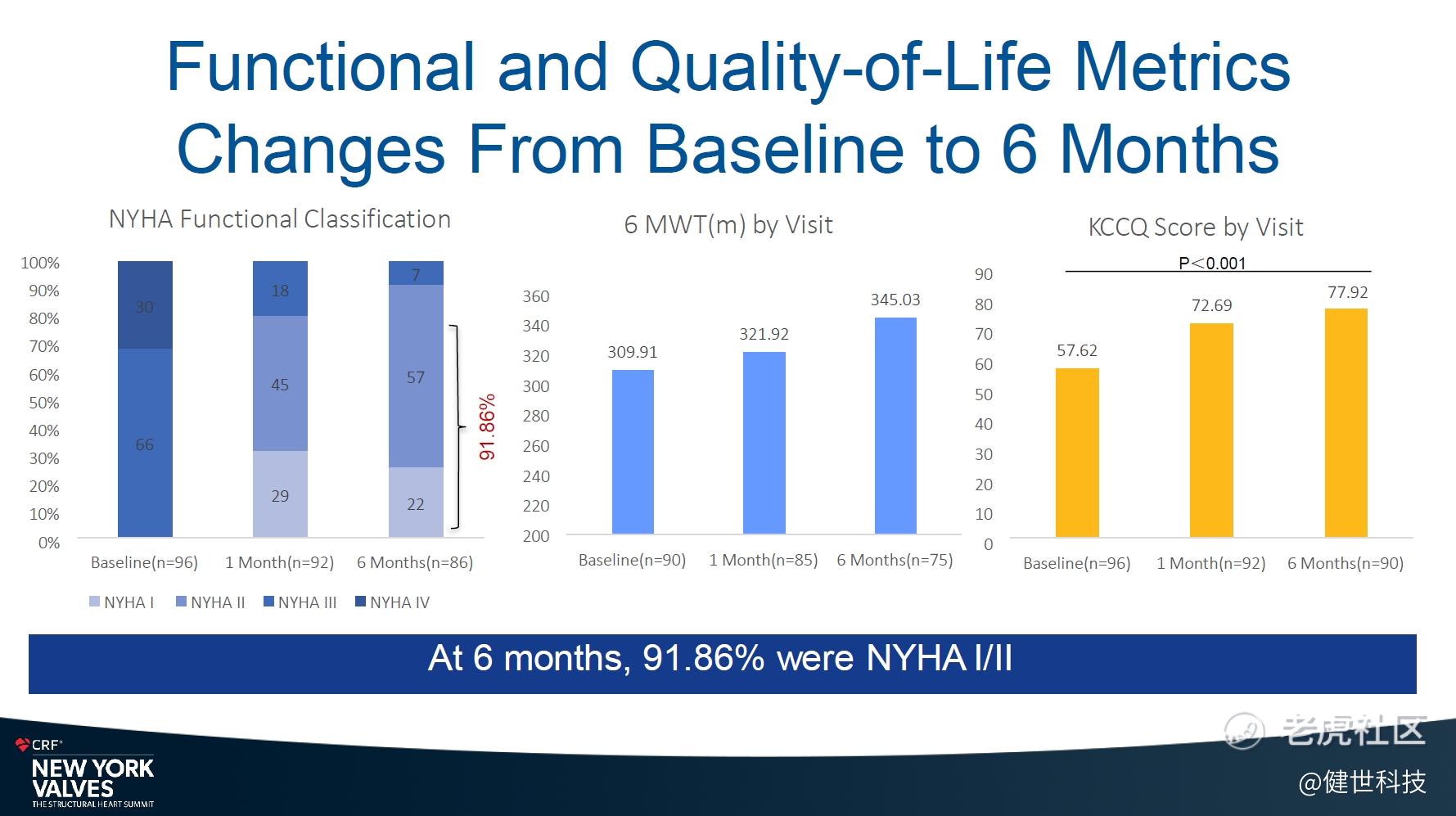
In terms of NYHA cardiac function improvement, 84.43% of patients improved from pre-procedure NYHA class III/IV to class I/II at 30 days, and 91.86% improved to NYHA class I/II at 6 months. In terms of quality of life (QoL), patients increased their Kansas City Cardiomyopathy Questionnaire (KCCQ) averaging score by 15 points at 30 days, and increased by 20 points at 6 months. The results indicate that the cardiac function and QoL of patients improved continuously.
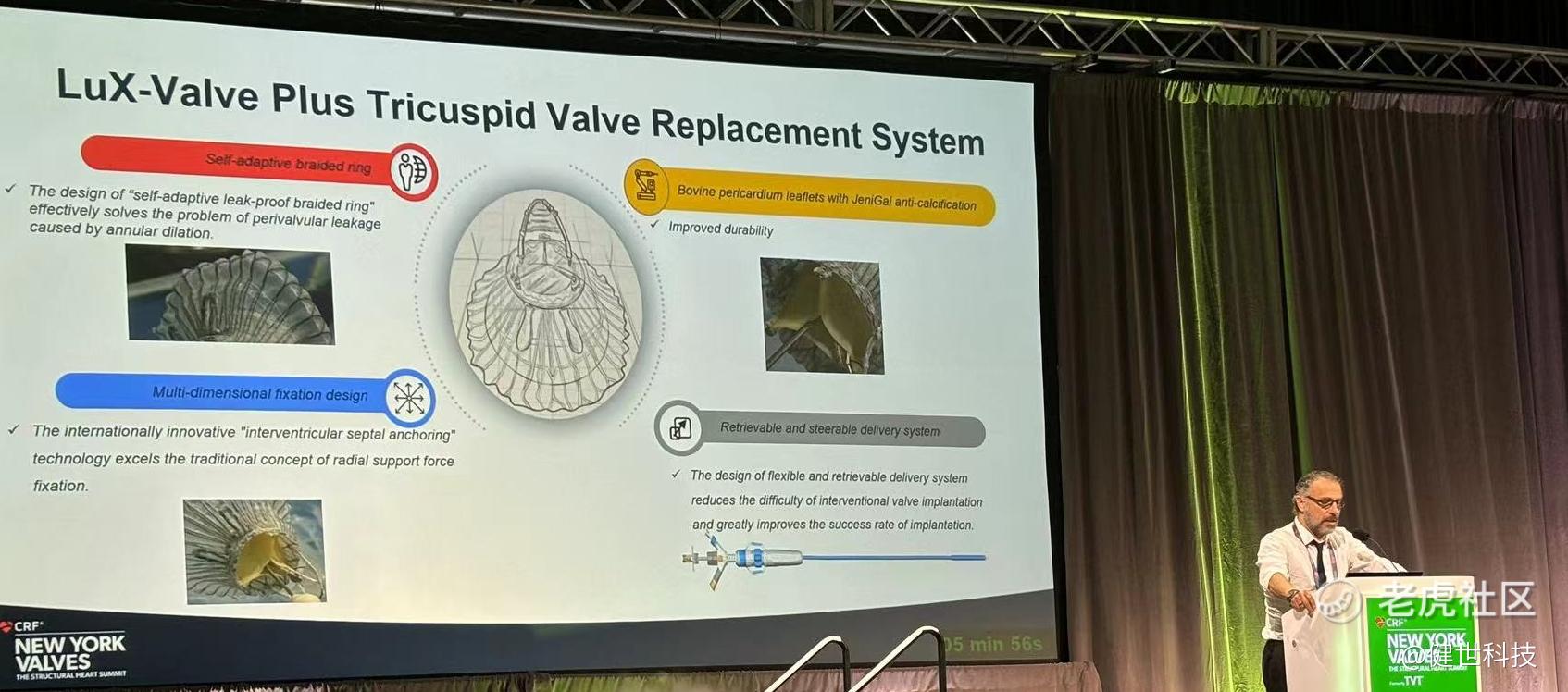
Prof. Granada concluded that LuX-Valve Plus system is a versatile TTVR device that does not depend on radial force for anchoring. Its innovative design (ventricular septal anchor & leaflet-grasping clips) provides redundant mechanisms for anchoring and stability. The multicenter, TRAVEL II study showed that LuX-Valve Plus system was safe and effective in achieving short delivery times, low 30-day composite event rates, significant TR reduction and improvement in functional and QoL metrics at 6 months.
Prof. Fanglin Lu concluded that, LuX-Valve Plus intended to shorten the learning curve and decrease the difficulties of TR interventional treatment.
---------------------------------------------------------------------------------------------------------------------------------------------------------
Global Compassionate Use Results of LuX-Valve Plus were Officially Released at EuroPCR 2024, and were Shared again at New York Valves 2024
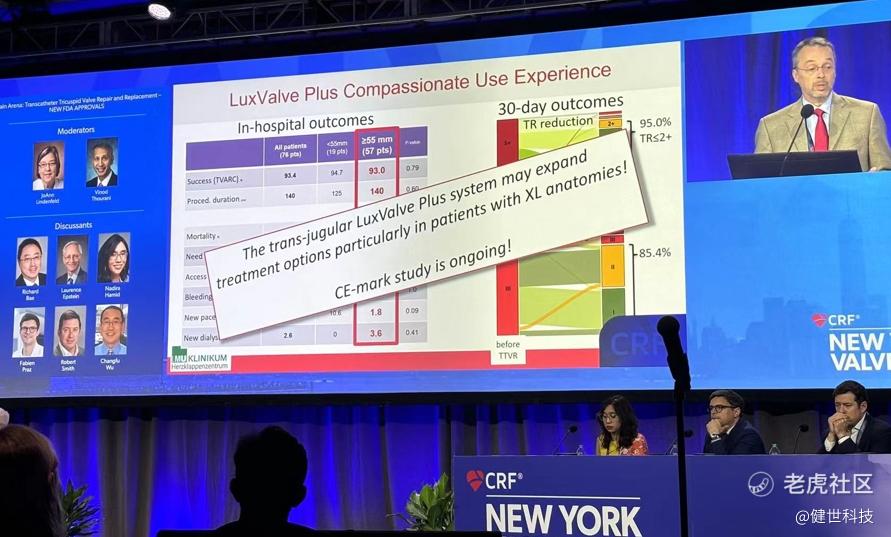
The global compassionate use outcomes of LuX-Valve Plus were released at EuroPCR 2024 by Prof. Jörg Hausleiter (Klinikum der Ludwig-Maximilians-Universität München, Munich, Germany). He later mentioned and appraised LuX-Valve Plus several times at New York Valves 2024. The results showed that the transjugular use of the LuX-Valve Plus system for TTVR is safe and it results in an efficacious TR reduction.
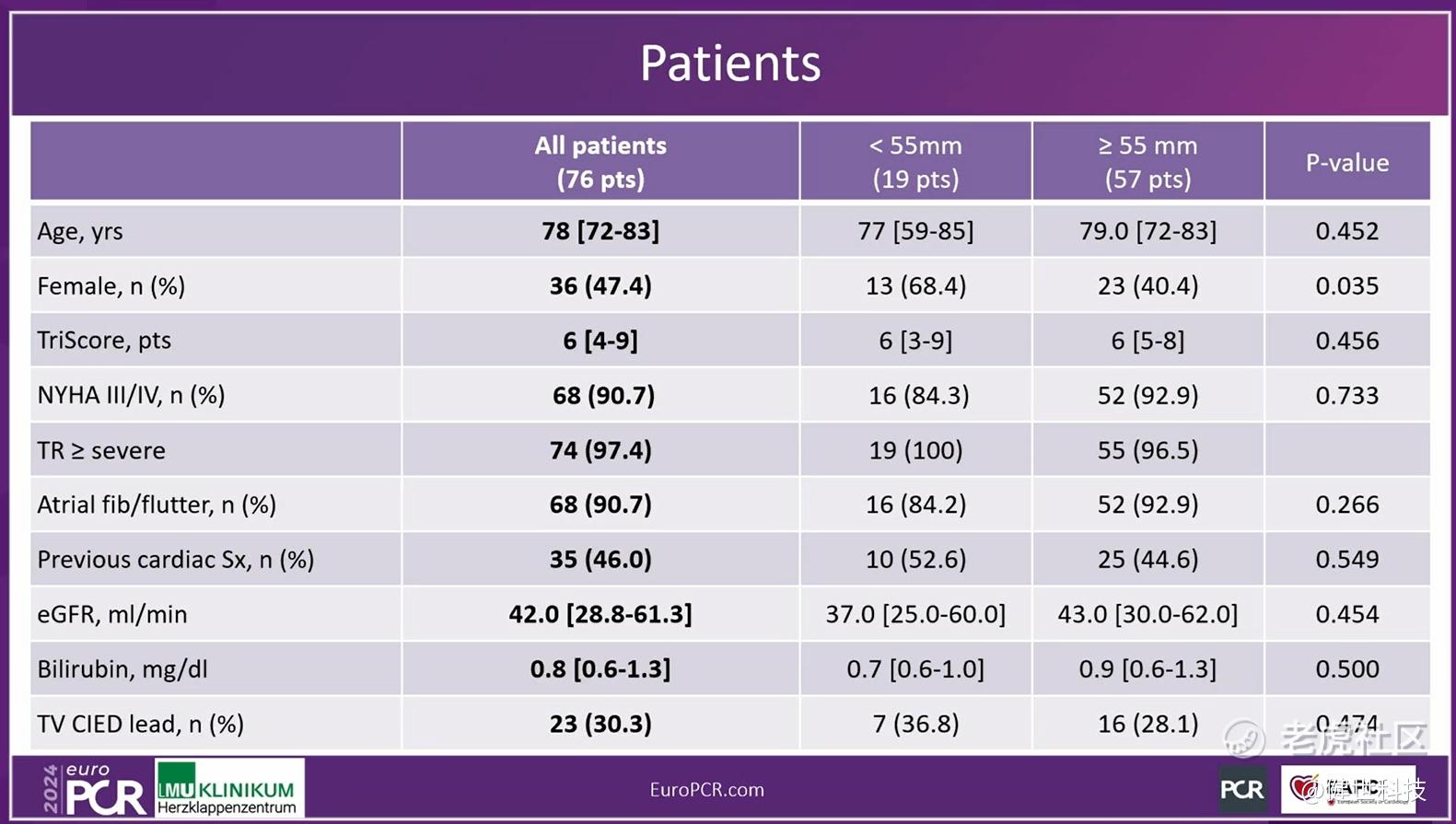
This compassionate use study was conducted among 15 sites internationally in recent years, and was widely spread in Europe, U.S. and Asia-Pacific region. It enrolled elderly patients from global population with high/extremely high surgical risk and severe TR or above. Since the patients in compassionate use were in poor conditions, the baseline was very challenging. The average age of the patients was 78 years old. 75% of patients have annulus diameter ≥55mm. 97.4% of patients have TR ≥3.
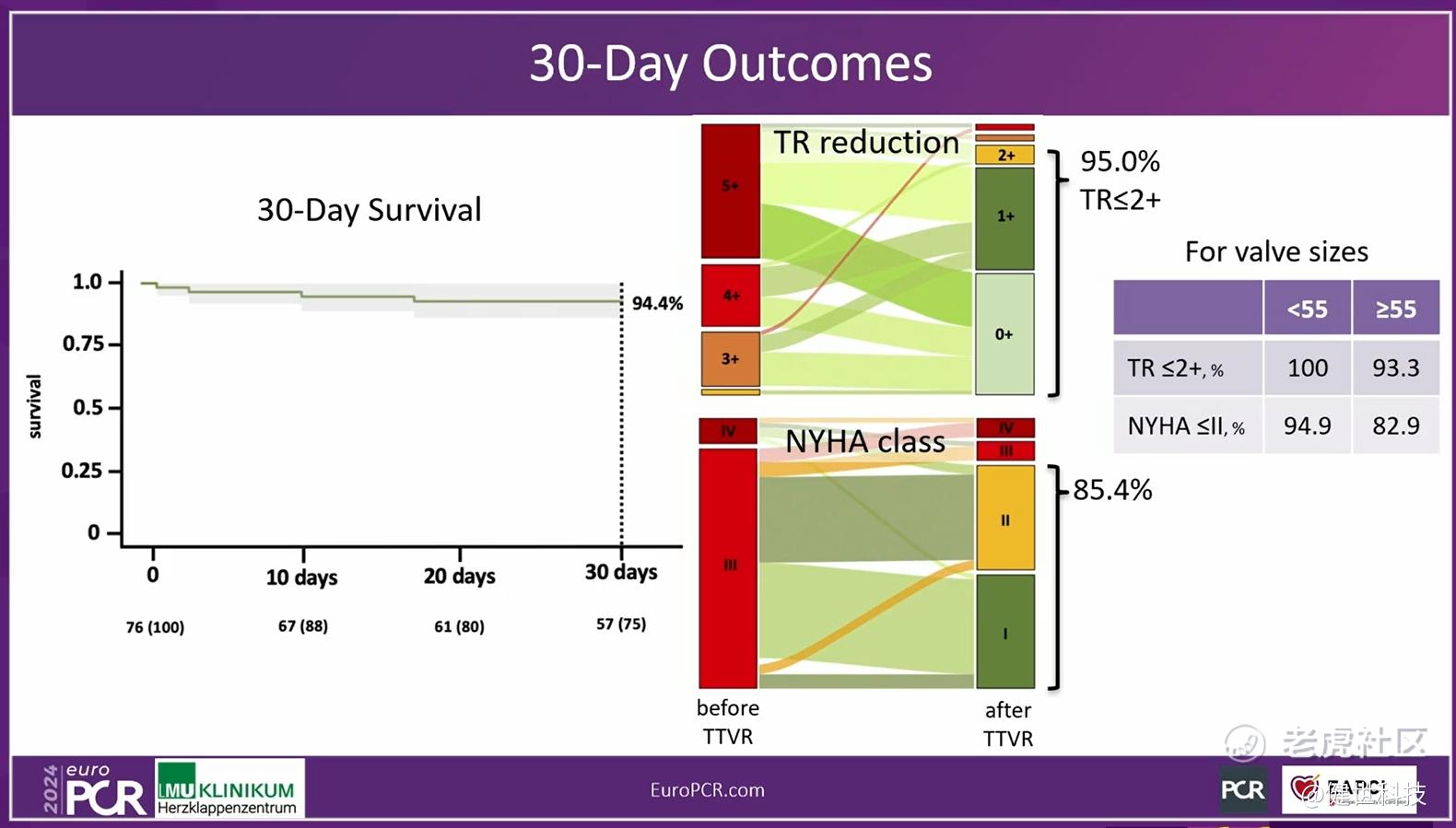
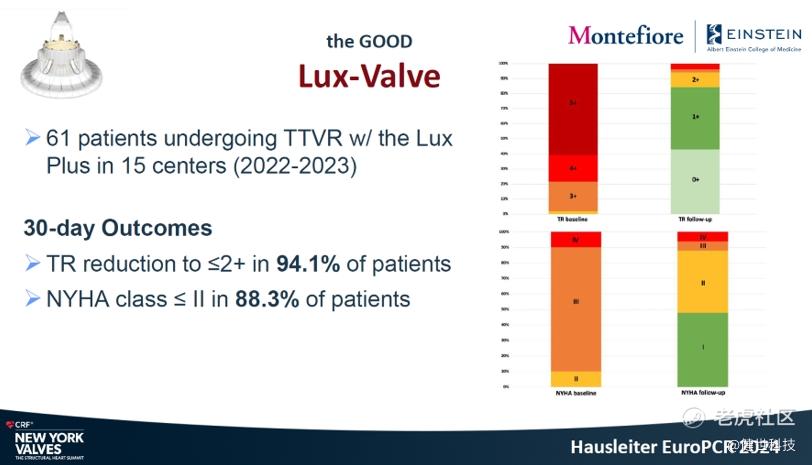
In-hospital and 30-day outcomes indicated outstanding treatment result. First, in-hospital TR grade reduced instantly. 92.2% of patients recovered to ≤2+. The incidence of new pacemaker implantation was only 3.9%. Then, 30-day outcomes showed TR grade reduced significantly, 95% of patients recovered to ≤2+. NYHA cardiac function improved continuously, 85.4% of patients improved to post-operative class I/II. Moreover, the echocardiographic findings showed the right heart/ventricle benefits as well.
Prof. Hausleiter concluded that, the compassionate use experience demonstrated that LuX-Valve Plus was safe and effective. The outcomes were comparable for patients receiving smaller (<55mm) or larger (≥55mm) valves, indicating that TTVR is also feasible in patients with advanced tricuspid disease characterized by large RV dimensions.
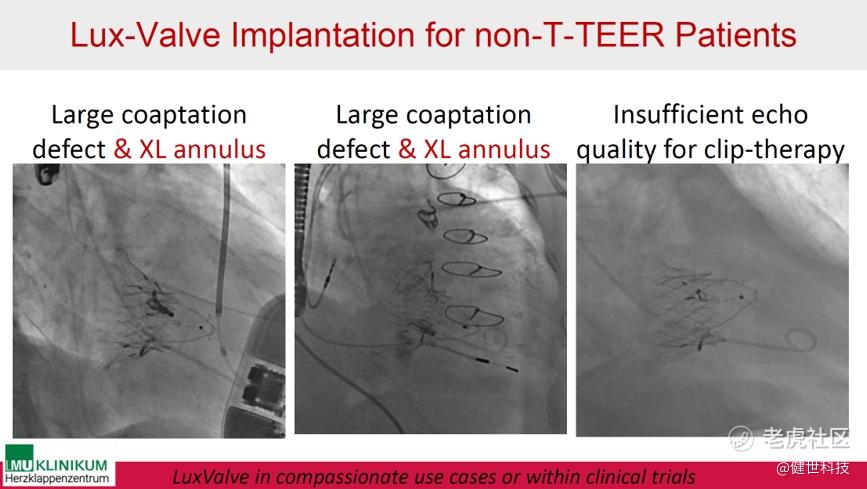
Later at the New York Valves 2024, Prof. Hausleiter mentioned LuX-Valve Plus again. He introduced that it can be applicable for patients who are not suitable for T-TEER, such as large coaptation defect & XL annulus, and insufficient echo quality for clip-therapy. Furthermore, LuX-Valve Plus may expand treatment options and shows promising early results.
---------------------------------------------------------------------------------------------------------------------------------------------------------
Hong Kong Compassionate Use Results of LuX-Valve Plus were Shared at CHINA VALVE (HANGZHOU) 2024
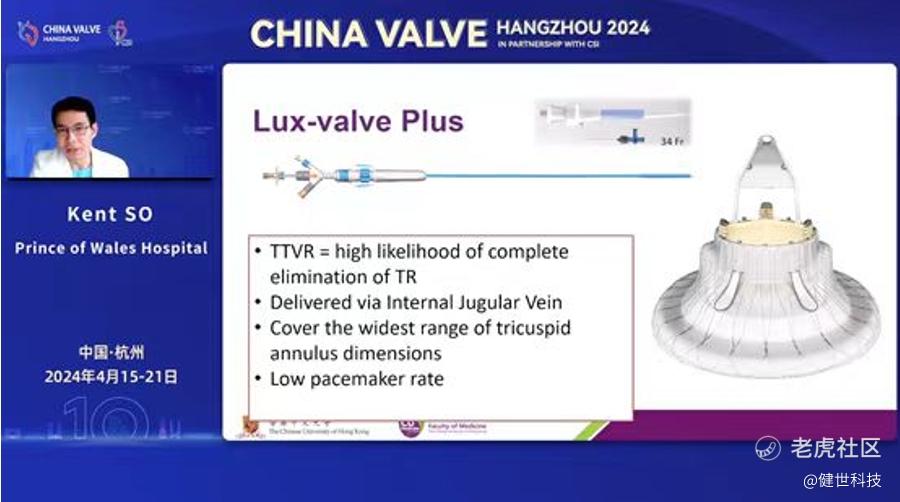
The Hong Kong early compassionate use experience of LuX-Valve Plus was shared by Prof. Kent So (苏泽宇教授, Prince of Wales Hospital, Chinese University of Hong Kong, Hong Kong, China) at CHINA VALVE (HANGZHOU) 2024. He pointed out that LuX-Valve Plus was a promising TTVR device, which can cover a wide range of tricuspid annulus dimensions with low pacemaker rate, and demonstrated good procedural and short-term result.
Prof. Kent So introduced that not all anatomies are suitable for T-TEER treatment or surgical operation because of the special anatomical structure of tricuspid valve and pathological changes accompanied. Therefore, TTVR has become the new clinical treatment option, which has a high likelihood of complete elimination of TR. LuX-Valve Plus is such kind of TTVR device which delivered via transjugular vein, can cover a wide range of tricuspid annulus dimensions with low pacemaker rate.
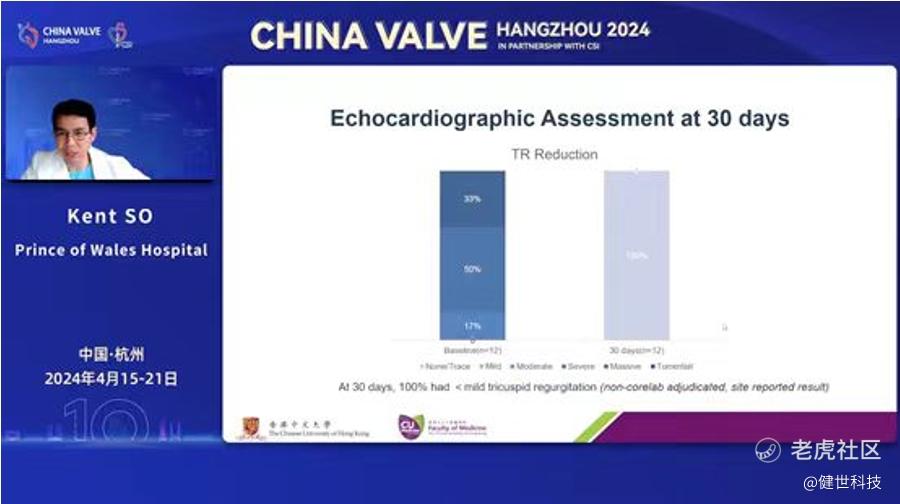
The HK early experience indicated that procedure success rate was 100%, and the procedural and 30-day outcomes showed that the incidences of all-cause mortality was 0%. The echocardiographic assessment at 30 days showed that 100% of the patients had their TR grade improved to none/trace. The safety and efficacy of LuX-Valve Plus were preliminarily validated one more time.
---------------------------------------------------------------------------------------------------------------------------------------------------------
Global Experience of Treating TR with LuX-Valve Plus were Shared and Discussed Multi Times in 2024H1
What Can LuX-Valve Plus Offer to Solve Current Clinical Challenges were Shared by Prof. Thomas Modine at New York Valves 2024
Experience with LuX-Valve Plus in Patient after TEER were Shared by Prof. Simon Lam at New York Valves 2024
The GOOD of LuX-Valve Plus were Shared by Prof. Azeem Latib at New York Valves 2024
Treating TR with LuX-Valve Plus were Shared by Prof. Lai Wei at Tapei Valve Summit 2024
LuX-Valve Plus Successfully Completed the First Clinical Implantation of Latin America in Brazil
---------------------------------------------------------------------------------------------------------------------------------------------------------
LuX-Valve Plus is about to Complete the Enrollment of CE Registration Clinical Trial in Europe
---------------------------------------------------------------------------------------------------------------------------------------------------------
LuX-Valve Plus Officially Commenced the Preparation of IDE Application
---------------------------------------------------------------------------------------------------------------------------------------------------------
JensClip Completed the Enrollment for Confirmatory Clinical Trial and One-month Follow-up
---------------------------------------------------------------------------------------------------------------------------------------------------------
Disclaimer
The information disclosed in this article is for academic communication purposes only and should not be construed as medical advice under any circumstances. We make no representations or warranties as to the accuracy, completeness or timeliness of all original, reproduced or shared content. For any medical device mentioned in this article, our company does not make any commitment and guarantee on its performance and clinical performance in diagnosis and treatment activities.
The regulatory status of LuX-Valve Plus is as follows: pending NMPA approval in China and in investigational clinical study under FDA and EMA.
免责声明:上述内容仅代表发帖人个人观点,不构成本平台的任何投资建议。